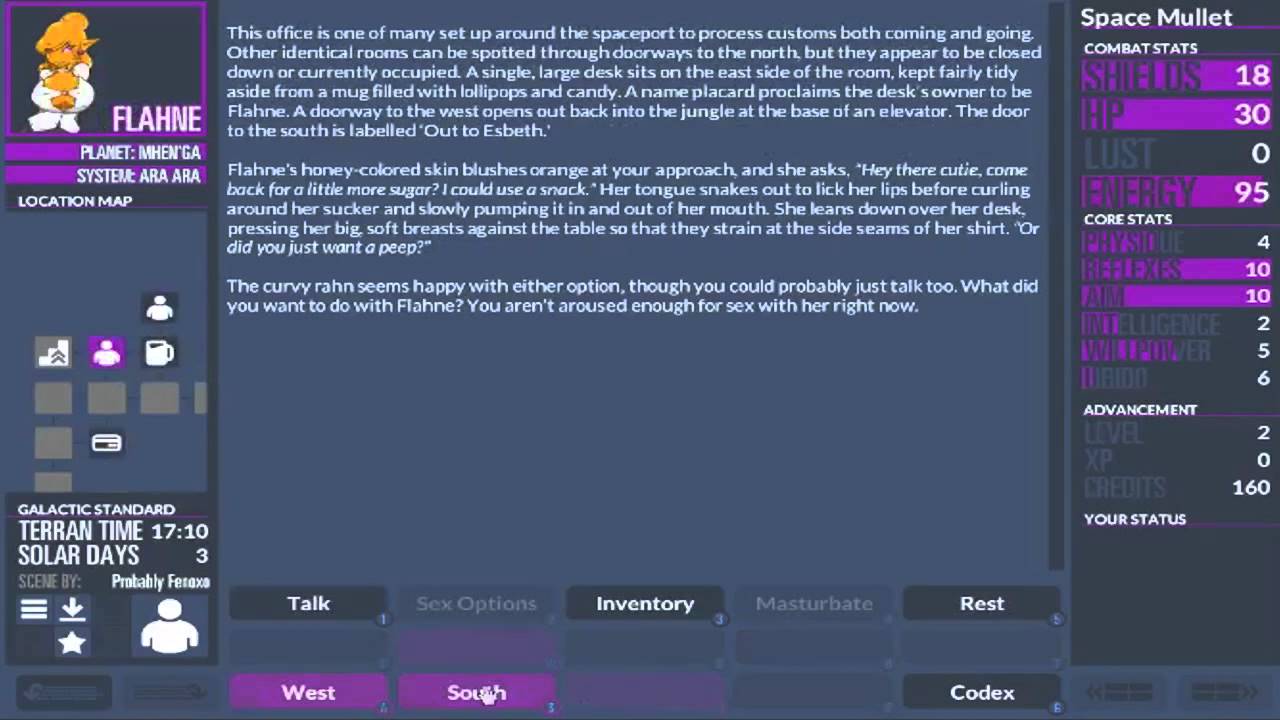
- 0.7.173 Changelog: Urbolg now has a few items he sells, a new talk topic, and a rare-rarity shield that can be retrieved from his office after completing the appropriate talk discussion.
- So I've been talking some time now about an upcoming project I'm looking to do, specifically the Trials in Tainted Space Bestiary. The goal of this project is to generate illustrations of each of the species found within the game, which later could be included in the game itself in a central database the PC would have within their ship's computer core.
- Max out your stats, buy a shield booster thing from the medic on the ant planet and also the armor buff, get a vial of grey goo to heal, buy the best gear you can afford and pray you land some crits or try to stun lock it. If you have flash bangs they help and tac goggles.
Play your favorite online game Among Us with free hacks and beat your opponents constantly. Trials In Tainted Space Cheats DOWNLOAD (Mirror #1) Tooltip 2. You gain the Selfish Shield champion's reaction and the touch of corruption devotion spell. Luckily i was able to figure something out using cheat engine.
White House press secretary Kayleigh McEnany tore into Brian Kemp, Georgia’s Republican governor, on Monday, calling him “despicable” and comparing him to Georgia-based voting rights advocate Stacey Abrams for refusing to call a special session to overturn the state’s presidential election results.
“Governor Kemp is no different than Stacey Abrams right now,” Ms McEnany, who is also a Trump campaign adviser, told Fox News’s Sean Hannity on Monday. “She did this consent decree, and by not doing this, Governor Kemp is Stacey Abrams and that is despicable.”
She echoed these claims on Twitter as well.
Right now, Governor Brian Kemp is no different than failed gubernatorial leftist Stacey Abrams.
It’s quite simple: Call a special legislative session to demand SIGNATURE MATCH — an electoral safeguard for 11/3/20 & for 1/5/21.
Sad to see INDISCERNIBLE RINOs & Dems!
— Kayleigh McEnany (@kayleighmcenany) December 8, 2020Ms McEnany and the president have wrongly insisted that a March court decision, in which Georgia agreed to standardized rules about judging signatures on mail-in ballots and notifying voters if there were problems, tainted the integrity of the election.
Nothing in the consent decree makes the vote less secure or prevents poll workers from scrutinizing signatures—in fact, they are legally required to.
The new policy came about after a lawsuit from the Democratic party, which argued the state’s signature-matching process was disproportionately disenfranchising minority voters. Ms Abrams is a Black woman herself, lending a racial undertone to Ms McEnany’s criticisms.
During the interview, Ms McEnany insisted her calls for a special session were not about overturning the election results, but the president has more or less explicitly said this is the point.

Video: U.S. Supreme Court rejects Republican challenge to Biden's Pennsylvania win (Reuters)
“The Republican Governor of Georgia refuses to do signature verification, which would give us an easy win,” he tweeted the same day of Ms McEnany’s interview. “What’s wrong with this guy? What is he hiding?”
The Republican Governor of Georgia refuses to do signature verification, which would give us an easy win. What’s wrong with this guy? What is he hiding?
— Donald J. Trump (@realDonaldTrump) December 7, 2020
The day before, he tweeted that Mr. Kemp, and his fellow Republican lieutenant governor Geoff Duncan, could “easily solve this mess, & WIN. Signature verification & call a Special Session. So easy!”
The president and his allies have rained criticism on Georgia officials after the state once again certified its election results for president elect Joe Biden after a series of recounts. Officials in the state, many of them Republicans, say the Democrat definitely won.

Mr Trump called governor Kemp over the weekend, reportedly to pressure him to call a special legislative session where the state’s GOP legislature could theoretically force the state’s Electoral College votes to go for Mr Trump, rather than reflecting the will of the state’s voters.
Trials In Tainted Space Cheat Codes
The governor and his lieutenant rejected calls for a special session in a statement on Sunday.
'Any attempt by the legislature to retroactively change that process for the November 3rd election would be unconstitutional and immediately enjoined by the courts, resulting in a long legal dispute and no short-term resolution,' they said.
Georgia’s secretary of state, also a Republican, echoed these comments that same day, saying, “They would be nullifying the will of the people.”
So far, legal challenges from the president’s allies trying to overturn the election have been unsuccessful in Georgia and elsewhere.
Following the election, despite no evidence of systemic fraud or voting irregularities, Georgia Republicans began considering whether to make it harder to vote by mail, which rights advocates and Democrats argue wouldn’t be necessary and could disenfranchise voters.

The president has repeatedly attacked mail-in voting and the Postal Service for causing his loss, even though he voted by mail himself this election.
Governor Kemp, now the focus of so much Republican ire for not putting his thumb on the scale for Republicans, was criticized for the exact opposite thing in 2018, when he beat Ms Abrams in the race for governor. Prior to his election, he removed thousands of people from Georgia’s voter rolls, many of them Black, while he was secretary of state presiding over his own election, which a subsequent investigations found kept thousands of active voters from registering their pick.
Hear what Dr. Buttar and Robert Scott Bell have to say about this article on the April 26, 2017 Medical Rewind Show.
Agents of the Food and Drug Administration know better than anyone else just how bad scientific misbehavior can get. Reading the FDA’s inspection files feels almost like watching a highlights reel from a Scientists Gone Wild video. It’s a seemingly endless stream of lurid vignettes—each of which catches a medical researcher in an unguarded moment, succumbing to the temptation to do things he knows he really shouldn’t be doing. Faked X-ray reports. Forged retinal scans. Phony lab tests. Secretly amputated limbs. All done in the name of science when researchers thought that nobody was watching.
That misconduct happens isn’t shocking. What is: When the FDA finds scientific fraud or misconduct, the agency doesn’t notify the public, the medical establishment, or even the scientific community that the results of a medical experiment are not to be trusted. On the contrary. For more than a decade, the FDA has shown a pattern of burying the details of misconduct. As a result, nobody ever finds out which data is bogus, which experiments are tainted, and which drugs might be on the market under false pretenses. The FDA has repeatedly hidden evidence of scientific fraud not just from the public, but also from its most trusted scientific advisers, even as they were deciding whether or not a new drug should be allowed on the market. Even a congressional panel investigating a case of fraud regarding a dangerous drug couldn’t get forthright answers. For an agency devoted to protecting the public from bogus medical science, the FDA seems to be spending an awful lot of effort protecting the perpetrators of bogus science from the public.
Much of my research has to do with follies, foibles, and fraud in science, and I knew that the FDA wasn’t exactly bending over backward to correct the scientific record when its inspectors found problems during clinical trials. So as part of my investigative reporting class at New York University, my students and I set out to find out just how bad the problem was—and how much important information the FDA was keeping under wraps.
The silence is unbroken even when the FDA itself seems shocked at the degree of fraud and misconduct.
We didn’t have to search very hard to find FDA burying evidence of research misconduct. Just look at any document related to an FDA inspection. As part of the new drug application process, or, more rarely, when the agency gets a tipoff of wrongdoing, the FDA sends a bunch of inspectors out to clinical sites to make sure that everything is done by the book. When there are problems, the FDA generates a lot of paperwork—what are called form 483s, Establishment Inspection Reports, and in the worst cases, what are known as Warning Letters. If you manage to get your hands on these documents, you’ll see that, most of the time, key portions are redacted: information that describes what drug the researcher was studying, the name of the study, and precisely how the misconduct affected the quality of the data are all blacked out. These redactions make it all but impossible to figure out which study is tainted. My students and I looked at FDA documents relating to roughly 600 clinical trials in which one of the researchers running the trial failed an FDA inspection. In only roughly 100 cases were we able to figure out which study, which drug, and which pharmaceutical company were involved. (We cracked a bunch of the redactions by cross-referencing the documents with clinical trials data, checking various other databases, and using carefully crafted Google searches.) For the other 500, the FDA was successfully able to shield the drugmaker (and the study sponsor) from public exposure.
A redacted letter sent by the FDA to a clinical investigator in 2012. Read letter here
It’s not just the public that’s in the dark. It’s researchers, too. And your doctor. As I describe in the current issue of JAMA Internal Medicine, my students and I were able to track down some 78 scientific publications resulting from a tainted study—a clinical trial in which FDA inspectors found significant problems with the conduct of the trial, up to and including fraud. In only three cases did we find any hint in the peer-reviewed literature of problems found by the FDA inspection. The other publications were not retracted, corrected, or highlighted in any way. In other words, the FDA knows about dozens of scientific papers floating about whose data are questionable—and has said nothing, leaving physicians and medical researchers completely unaware. The silence is unbroken even when the FDA itself seems shocked at the degree of fraud and misconduct in a clinical trial.
Such was the case with the so-called RECORD 4 study. RECORD 4 was one of four large clinical trials that involved thousands of patients who were recruited at scores of clinical sites in more than a dozen countries around the world. The trial was used as evidence that a new anti-blood-clotting agent, rivaroxaban, was safe and effective. The FDA inspected or had access to external audits of 16 of the RECORD 4 sites. The trial was a fiasco. At Dr. Craig Loucks’ site in Colorado, the FDA found falsified data. At Dr. Ricardo Esquivel’s site in Mexico, there was “systematic discarding of medical records” that made it impossible to tell whether the study drug was given to the patients. At half of the sites that drew FDA scrutiny—eight out of 16—there was misconduct, fraud, fishy behavior, or other practices so objectionable that the data had to be thrown out. The problems were so bad and so widespread that, contrary to its usual practice, the FDA declared the entire study to be “unreliable.” Yet if you look in the medical journals, the results from RECORD 4 sit quietly in The Lancet without any hint in the literature about falsification, misconduct, or chaos behind the scenes. This means that physicians around the world are basing life-and-death medical decisions on a study that the FDA knows is simply not credible.
It’s not just one study, either. The FDA found major problems with sites involved in the other three clinical trials that were used to demonstrate rivaroxaban’s safety and effectiveness. RECORD 2, for example, was nearly as awful as RECORD 4: Four out of 10 sites that the FDA inspected showed evidence of misconduct, or other issues grave enough to render the site’s data worthless—including clear evidence of data falsification at one site. In aggregate, these problems raise serious doubts about the quality of all four key rivaroxaban studies—and, by extension, doubts about how seriously we should take the claim that rivaroxaban is safe and effective. The FDA is keeping mum, even as wrongful-death lawsuits begin to multiply.
The FDA’s failure to notify the public is not merely a sin of omission. In March 2009, the FDA convened a committee of outside scientific experts to mull the “robustness and meaningfulness” of the results from the four rivaroxaban trials, RECORDs 1, 2, 3, and 4. (The agency regularly calls in advisers to get advice, or, more cynically, to get cover, about a decision the agency has to make.) When the agency briefed the committee, it was (to put it mildly) coy about the problems it was finding. It said only that inspectors had found “significant issues” at two clinical sites involved in the RECORD 4 study—and that data from one of them was included in the analysis. Inspections were still ongoing, so it’s not easy to say precisely what the agency knew at that point, but it’s clear that the FDA wasn’t admitting to everything it knew. A bunch of inspections had been completed a month prior to the meeting, and we know for certain that the agency was fully aware of major issues beyond the two it revealed to the advisory committee. In a memo dated three days before the advisory committee meeting convened, the FDA detailed “falsification of data by a subinvestigator” at a RECORD 2 site. The advisory committee was not told.
By itself, this might seem like a miscommunication or an oversight, but the FDA has a history of not notifying the public about the misconduct it finds. About a decade ago, the agency got into trouble over a newly approved antibiotic, Ketek. Inspectors had found extensive problems (including fraud) affecting key clinical trials of the drug. Yet the agency did its best to hide the problems from even its most trusted advisers. As David Ross, the FDA official in charge of reviewing Ketek’s safety, put it, “In January 2003, over reviewers’ protests, FDA managers hid the evidence of fraud and misconduct from the advisory committee, which was fooled into voting for approval.” However, when the reports of misconduct at one clinical site began appearing in the press—along with stories of liver damage and blurred vision associated with the new drug—Congress stepped in, demanding information from the agency about the fraud.
Trials In Tainted Space Wiki
But even the Senate couldn’t wring key information about the misconduct out of the FDA. “Every excuse under the sun has been used to create roadblocks,” complained an indignant Sen. Charles Grassley, “even in the face of congressional subpoenas requesting information and access to FDA employees.” The head of the FDA, Andrew von Eschenbach, attempted to explain to Congress why the agency didn’t tell its advisory committee about the problems in the Ketek study: “After considering the fact that the investigation results were preliminary … FDA decided to hold the Advisory Committee meeting as planned …” without notifying the committee of the potential problems. But Rep. Bart Stupak quickly pointed to an email, which, he argued, contradicted von Eschenbach’s testimony. “So either you are not being forthright with us, when I believe you are, but whoever is doing your work is trying to lead this committee down the wrong path.” And the correct path showed that siteaftersite involved in study 3014, as well as other key Ketek studies, were tainted as well.
In the decade since the Ketek affair, it’s hard to see any change in behavior by the agency. On occasion, the FDA has even actively approved and promoted statements about drugs that, according to its own inspectors, are based upon falsehoods. At the end of 2011, the FDA learned that an audit of a Chinese site involved in a key clinical trial of a different anti-clotting agent, apixaban, had turned up evidence of fraud: Personnel had apparently been fiddling with patient records. Worse yet, the fraud appeared to invalidate one key finding of the study. Just three months earlier, the researchers running the trial proudly announced in the New England Journal of Medicine that there was a “significant reduction in mortality” among patients who took apixaban compared with those who took the old standby, warfarin. Alas, the moment you exclude the data from the Chinese fraud site, as per standard FDA procedure, that statement went out the window. Yet look at the label for apixaban—the one approved by the FDA after the fraud was discovered—and you read that “treatment resulted in a significantly lower rate of all-cause death … than did treatment with warfarin,” backed up by the data set with the Chinese site included. In other words, the label is carrying a claim that the FDA knows is based upon fraud. In a written response to my questions on this subject, the FDA stated that, “The FDA extended the drug’s review period to address the concerns. However, the review team did conclude concluded [sic] that the data at that site and other sites in China did reflect meaningful clinical information; that was not what was considered unreliable.”
Trials In Tainted Space Best Shield Episodes
Again, this isn’t an isolated incident. I had previously encountered bogus data on FDA-approved labels when a colleague and I were looking into a massive case of scientific misconduct —a research firm named Cetero had been caught faking data from more than 1,400 drug trials. That suddenly worthless data had been used to establish the safety or effectiveness of roughly 100 drugs, mostly generics, that were being sold in the United States. But even after the agency exposed the problem, we found fraud-tainted data on FDA-approved drug labels. (The FDA still maintains its silence about the Cetero affair. To this day, the agency refuses to release the names of the 100-odd drugs whose approval data were undermined by fraud.)
And the FDA covers up drug-related misconduct in other, more subtle ways, too. For example, the agency publishes the canonical listing of generic drugs in the United States, known as the “Orange Book.” Prescription drugs in this book are often given what’s called a “therapeutic equivalence code.” This code is a two-letter designation that signals the quality of the scientific evidence that a generic is “bioequivalent” to the name-brand drug. The code “AB,” for example, tells pharmacists and physicians that there are solid scientific studies proving that bioequivalence. Another code, “BX,” signals that there isn’t sufficient data to prove the generic is bioequivalent to the name brand.
Trials In Tainted Space Azra
READ THE REMAINDER OF THIS ARTICLE………CLICK HERE
